CANDIPLUS®
Candiplus® in the treatment of vaginal mycosis
ProFem's current lead product Candiplus® is a new, patent-protected drug for the local treatment of frequently recurring vaginal fungal infections. The clinical efficacy of the new combination product Candiplus® is mediated by a completely new mechanism, which is based on synergistic effects of the active ingredient components. According to the study results available to date, the onset of action is rapid and effective, while at the same time frequent relapses and chronicity are prevented.
UNIQUE BENEFITS FOR PATIENTS
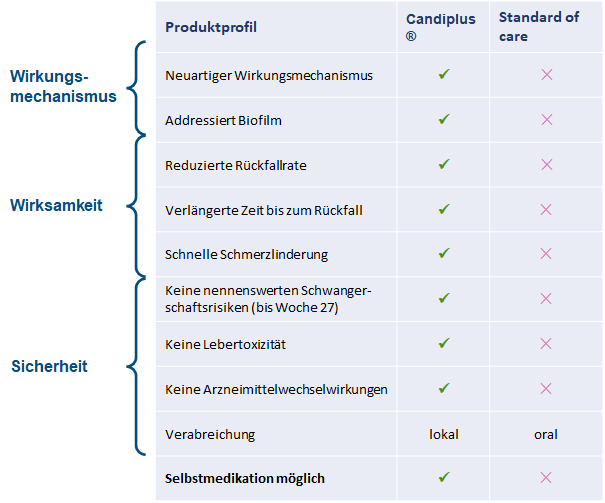
Candiplus® shows significant advantages over currently established therapies.
SCIENTIFIC BACKGROUND
Chronic inflammatory processes and the accompanying expression of cellular adhesion molecules play a central role both in the transition of harmless (saprophytic) candida spores to the disease-causing candida hyphae and in the formation of biofilms. In addition, specific resistance mechanisms develop in biofilms, which lead to the failure of conventional therapeutic approaches and subsequently to the clinical pattern of recurrent disease episodes. In Candiplus® , for the first time in the treatment of chronic fungal infections, adhesion and invasion are specifically blocked, resistance mechanisms in the biofilm are overcome and the interaction between microorganism and host is normalized.
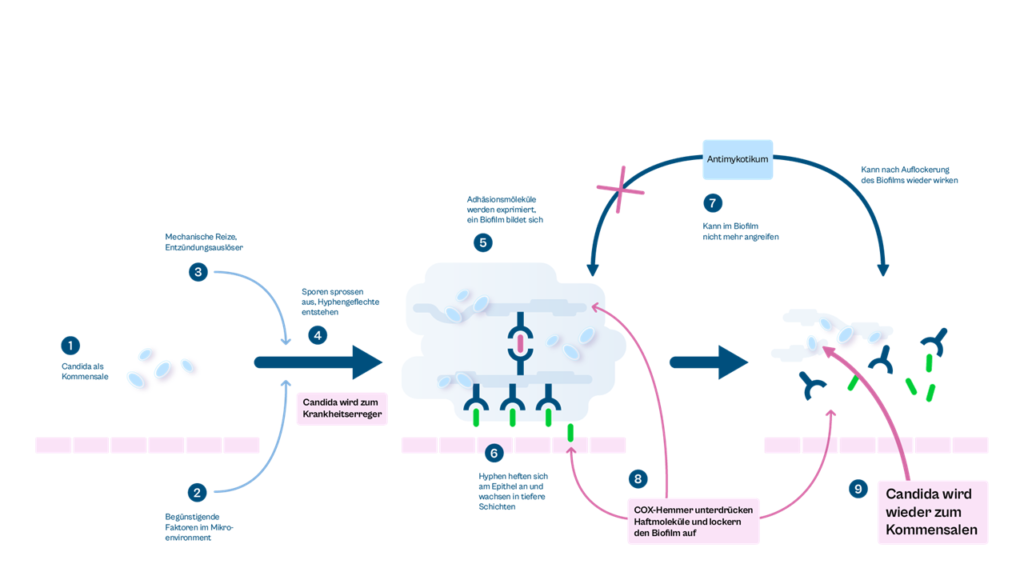
After the transition to the expansive growth form, the Candida yeast continuously produces inflammatory mediators with which the disease-causing process is constantly maintained. Conventional drugs that attack fungal growth temporarily inhibit the disease by suppressing fungal growth, but usually do not lead to complete regression of the microorganism to its initial saprophytic (peaceful) state. Candiplus® is the first drug worldwide to treat chronic infections causally and comprehensively. It is unique in its mode of action and has no comparable competitor. The implementation of this concept in Candiplus® thus results in a first-in-class drug.
CHRONIC VAGINAL FUNGUS
An unsolved medical problem
Hundreds of millions of women worldwide suffer from sporadic episodes of vaginal yeast infections (VVC). The incidence of this condition is reported to be 25-40 % of the female population aged 15-65.1
However, about 6 % (3 - 8%) of all women in this age group suffer frequently (several times per year) or chronically from such fungal infections (recurrent vulvovaginal candidiasis, RVVC). This corresponds to a total number of about 150 million women.2
The recurrent/chronic course of this disease is associated with severe impairment of subjective well-being and currently has no sustainable cure.3 The quality of life of the affected women is therefore massively restricted - often for years. Therapeutic options have not expanded over the decades. In particular, therapeutic approaches to date have not taken into account the fact that chronic recurrent vulvovaginal candidiasis is a biofilm-associated disease, whereby novel resistance mechanisms develop and conventional therapeutic strategies are only partially effective.
1 Foxman et al (2013) Am Soc Col Cer Path, 17(3):340-45.
2 Denning et al, Global prevalence of recurrent and chronic vulvovaginal candidiasis, www.ecsmid.org
3 Samuel Aballéa, Florent Guelfucci, et al, Subjective health status and health-related quality of life among women with Recurrent Vulvovaginal Candidosis (RVVC) in Europe and the USA; Health and Quality of Life Outcomes 2013 11:169. doi.org/10.1186/1477-7525-11-169; © Aballéa et al.; licensee BioMed Central Ltd. 2013
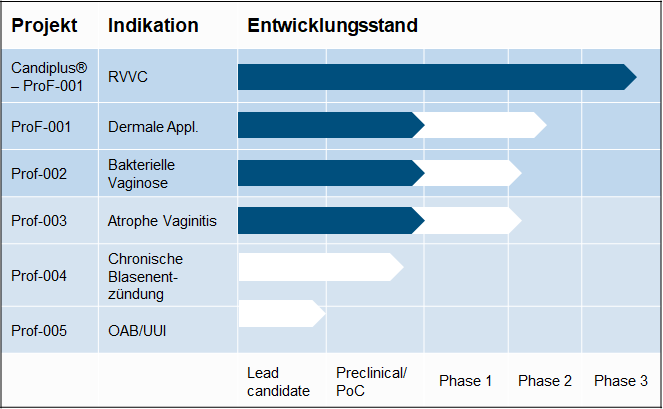
PIPELINE